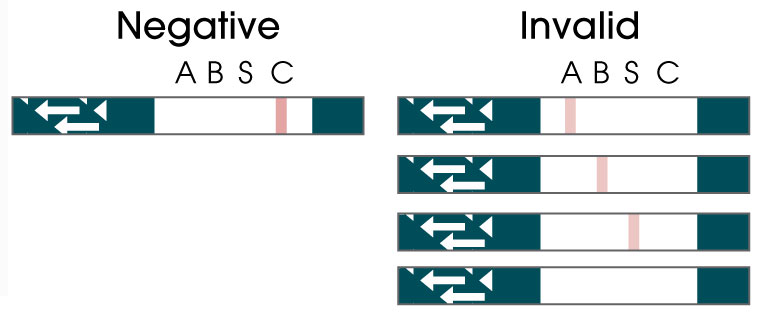
Covid-19/Flu A&B
OSOM® Flu SARS-CoV-2 Combo Test

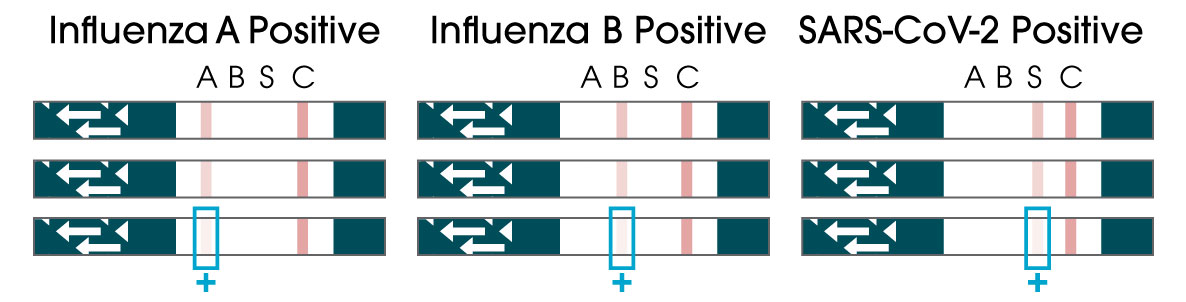
From only one sample, the OSOM® Flu SARS-CoV-2 Combo Test simultaneously detects and differentiates between COVID-19, Flu A, and Flu B, allowing healthcare providers to make more informed decisions when treating patients who are symptomatic for common viral infections.
Sample Type: Anterior nasal sample
Package Insert
In the USA, this product has not been FDA cleared or approved, but has been authorized by FDA under an Emergency Use Authorization. This product has been authorized only for the detection of proteins from SARS-CoV-2, influenza A and influenza B, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.

From only one sample, the OSOM® Flu SARS-CoV-2 Combo Test simultaneously detects and differentiates between COVID-19, Flu A, and Flu B, allowing healthcare providers to make more informed decisions when treating patients who are symptomatic for common viral infections.
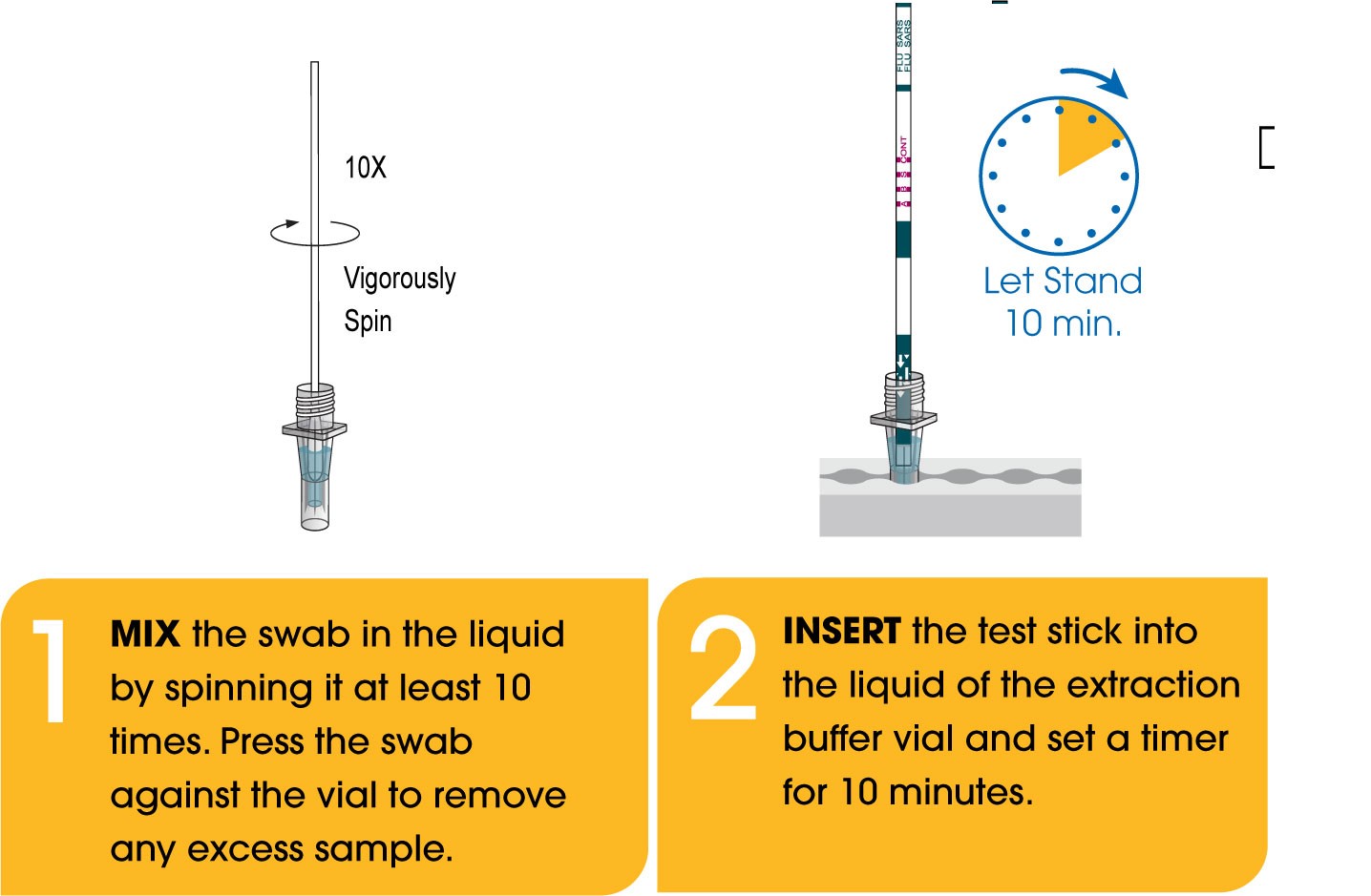
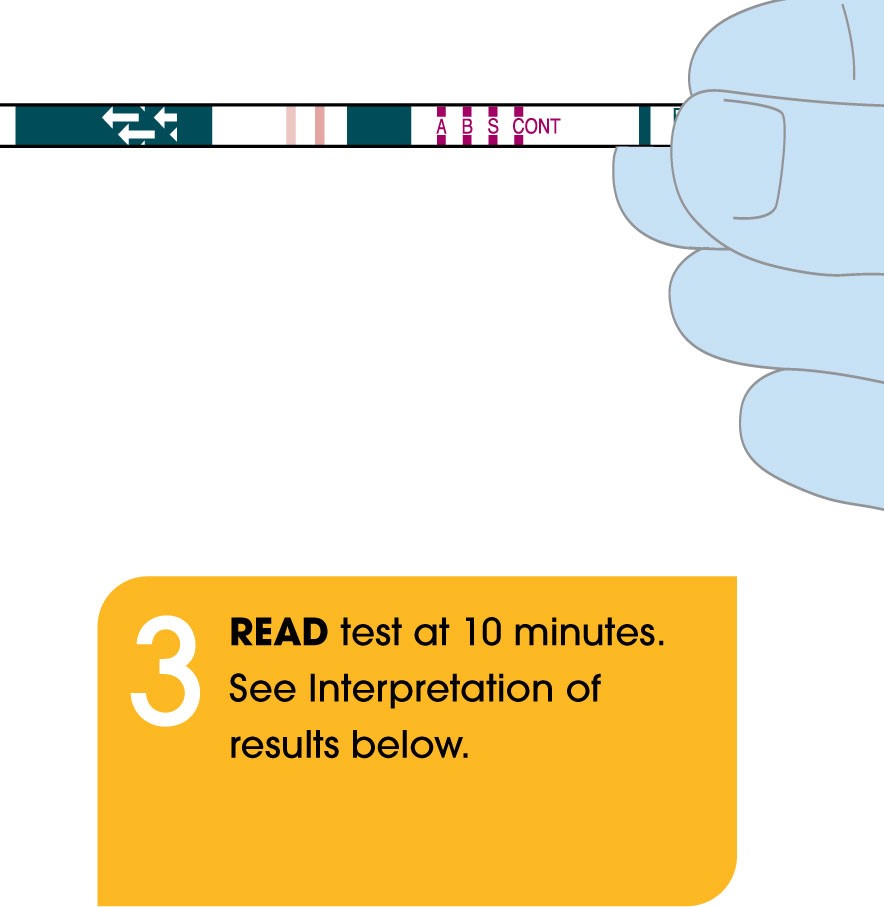
- Rapid: Differentiate between three viral infections (COVID-19, Flu A, & Flu B) in only 10 minutes at the point-of-care.
- Reliable: Proven clinical performance to help you reduce the risk of misdiagnosis and inappropriate treatment.
- Cost Efficient: Unique QC Inside® feature that provides you with two additional tests for external quality control testing.
- Easy-to-Use: Ready-to-use extraction buffers contained in each sample collection tube, so you do not have to perform a rehydration or dispensing step.
- Peace-of-Mind: Manufactured in the USA so you can avoid delays from international product shipments.
Sample Type: Anterior nasal sample




Package Insert
In the USA, this product has not been FDA cleared or approved, but has been authorized by FDA under an Emergency Use Authorization. This product has been authorized only for the detection of proteins from SARS-CoV-2, influenza A and influenza B, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
OSOM - Covid-19, Flu A & B Combo Test, 25ct [ MP977-1080 ]
|
Price: $417.99
|
|

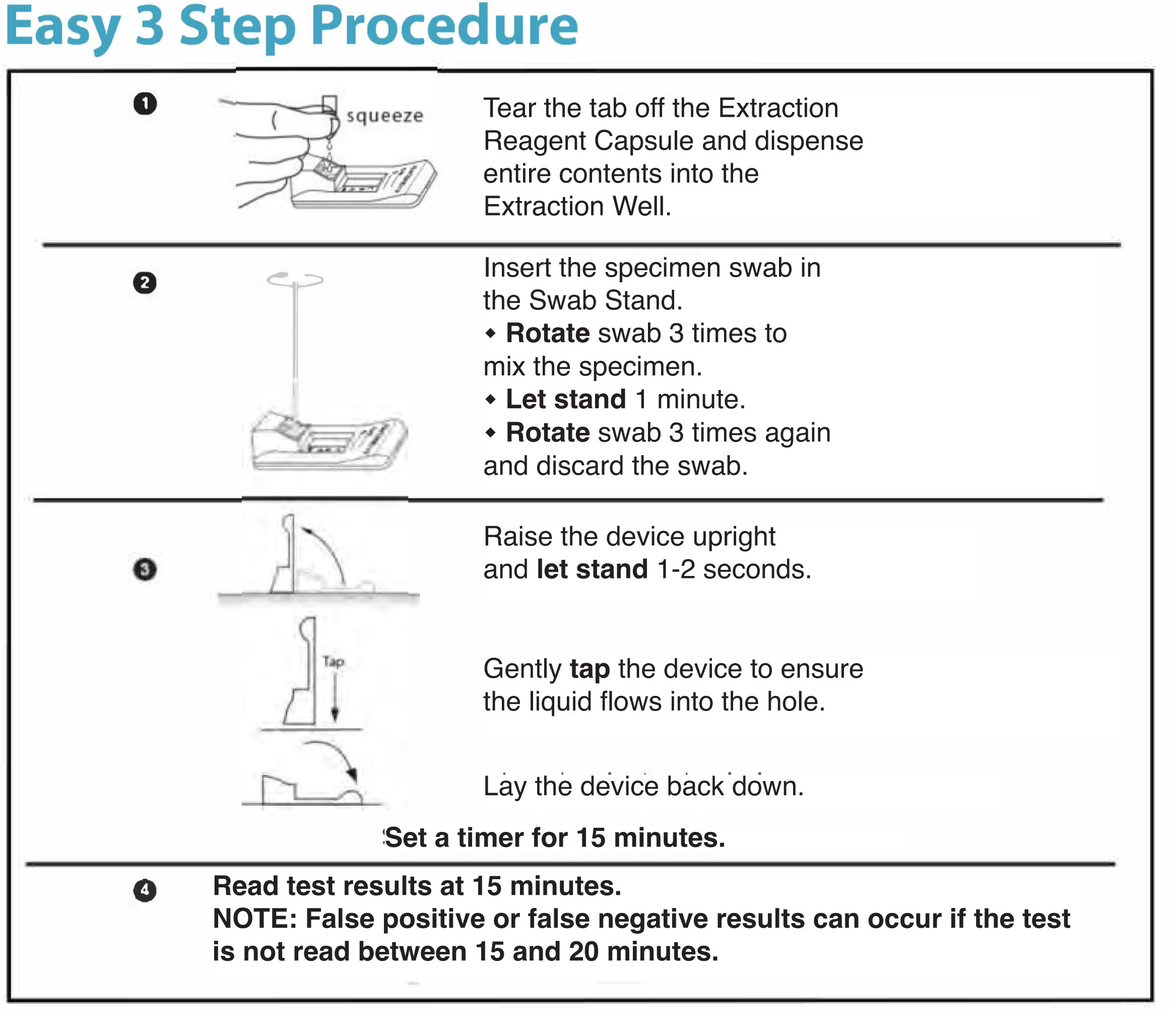
Status™ COVID-19/FLU A&B is a Rapid Immunoassay for the Simultaneous Direct Detection and Differential Diagnosis of SARS-CoV-2, Influenza Type A and Type B Antigen from anterior nasal and nasopharyngeal swab specimens
Status™ COVID-19/Flu test is a lateral flow immunoassay intended for the in vitro rapid, simultaneous qualitative detection and differentiation of nucleocapsid antigen from SARS-CoV2, influenza A and influenza B directly from anterior nasal and nasopharyngeal swab specimens obtained from individuals, who are suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider, within the first five days of onset of symptoms. Clinical signs and symptoms of respiratory viral infection due to SARS-CoV-2 and influenza can be similar. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests.
This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
- COVID-19
Nasopharyngeal − Sensitivity 93.1 %, Specificity 100%
- Flu A – Sensitivity 91.4%, Specificity 95.7%
- Flu B – Sensitivity 87.6%, Specificity 95.9%
- FDA Emergency Use Authorization (EUA)
- Visually read in 15 minutes
- Flocked nasopharyngeal swab for superior specimen collection and patient comfort
Kit Includes:
25 Test Devices
25 Extraction Reagents in Capsules
25 Sterile Swabs
1 Positive and 1 Negative Control Swabs
1 Package insert
1 Quick Reference Instruction
Product Insert
Covid-19/Flu A&B Rapid Test - Status (25ct) [ J390-ID33225 ]
|
Price: $534.99
|
|