Covid-19/Flu A&B Rapid Test - Status

Status™ COVID-19/FLU A&B is a Rapid Immunoassay for the Simultaneous Direct Detection and Differential Diagnosis of SARS-CoV-2, Influenza Type A and Type B Antigen from anterior nasal and nasopharyngeal swab specimens
Status™ COVID-19/Flu test is a lateral flow immunoassay intended for the in vitro rapid, simultaneous qualitative detection and differentiation of nucleocapsid antigen from SARS-CoV2, influenza A and influenza B directly from anterior nasal and nasopharyngeal swab specimens obtained from individuals, who are suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider, within the first five days of onset of symptoms. Clinical signs and symptoms of respiratory viral infection due to SARS-CoV-2 and influenza can be similar. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests.
This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
- COVID-19
Nasopharyngeal − Sensitivity 93.1 %, Specificity 100%
- Flu A – Sensitivity 91.4%, Specificity 95.7%
- Flu B – Sensitivity 87.6%, Specificity 95.9%
- FDA Emergency Use Authorization (EUA)
- Visually read in 15 minutes
- Flocked nasopharyngeal swab for superior specimen collection and patient comfort
Kit Includes:
25 Test Devices
25 Extraction Reagents in Capsules
25 Sterile Swabs
1 Positive and 1 Negative Control Swabs
1 Package insert
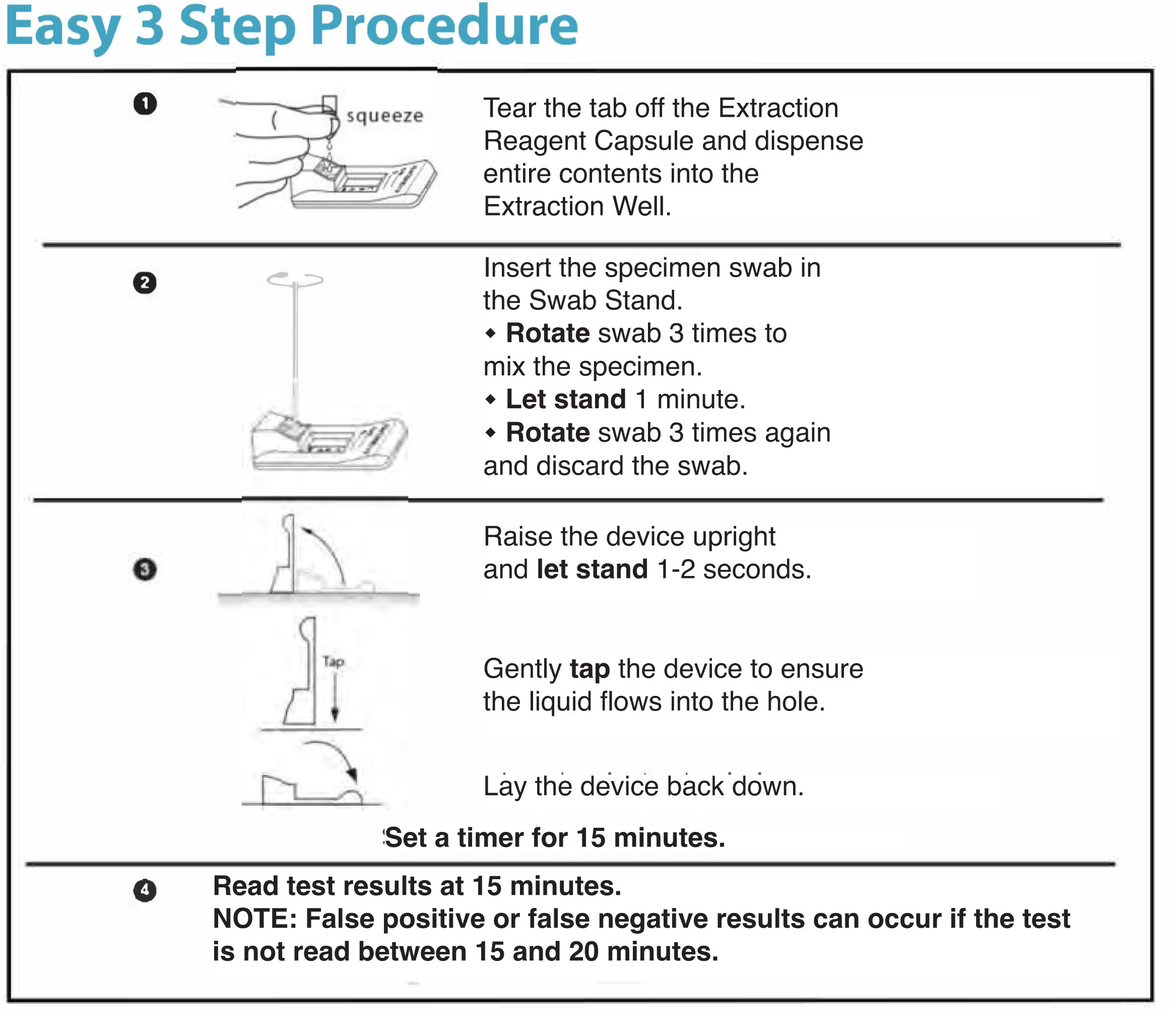
1 Quick Reference Instruction
Product Insert
Covid-19/Flu A&B Rapid Test - Status (25ct) [ J390-ID33225 ]
|
Price: $534.99
|
|
