OSOM - Covid-19, Flu A & B Combo Test, 25ct

OSOM® Flu SARS-CoV-2 Combo Test

From only one sample, the OSOM® Flu SARS-CoV-2 Combo Test simultaneously detects and differentiates between COVID-19, Flu A, and Flu B, allowing healthcare providers to make more informed decisions when treating patients who are symptomatic for common viral infections.
Sample Type: Anterior nasal sample
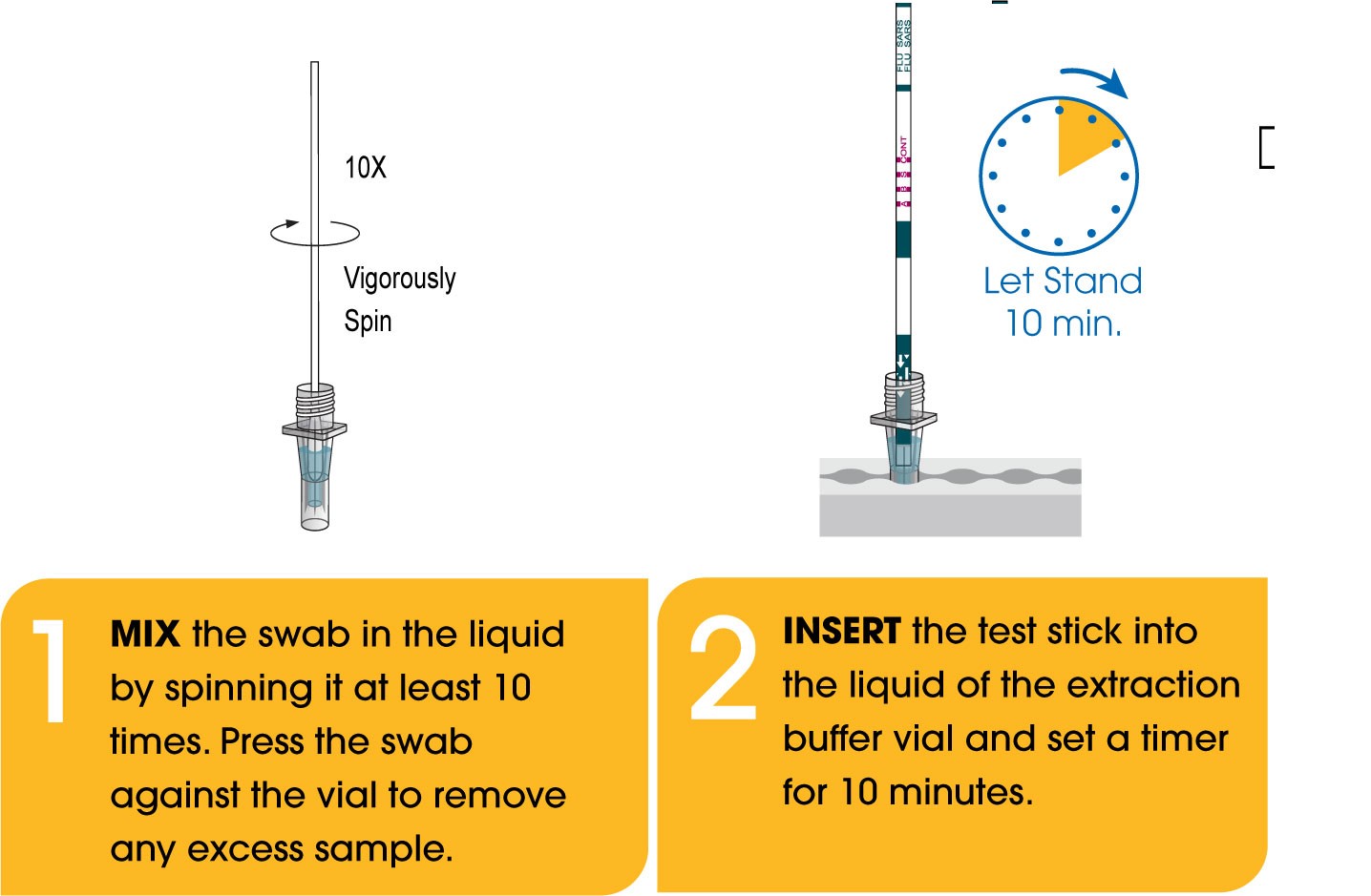
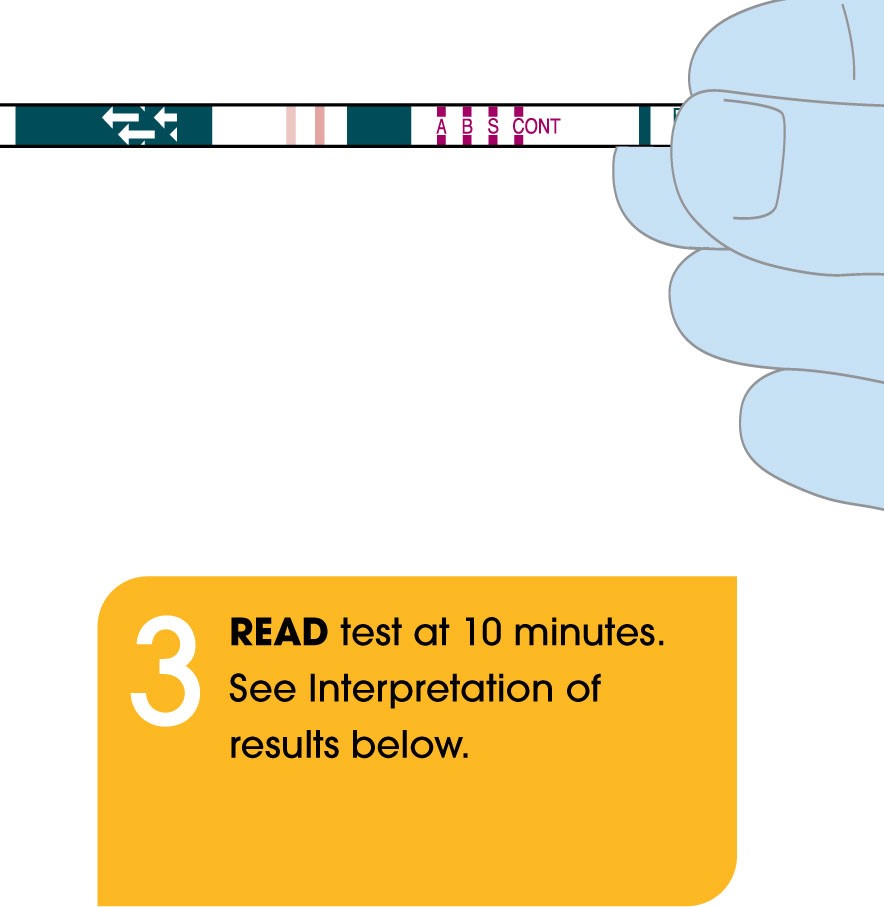
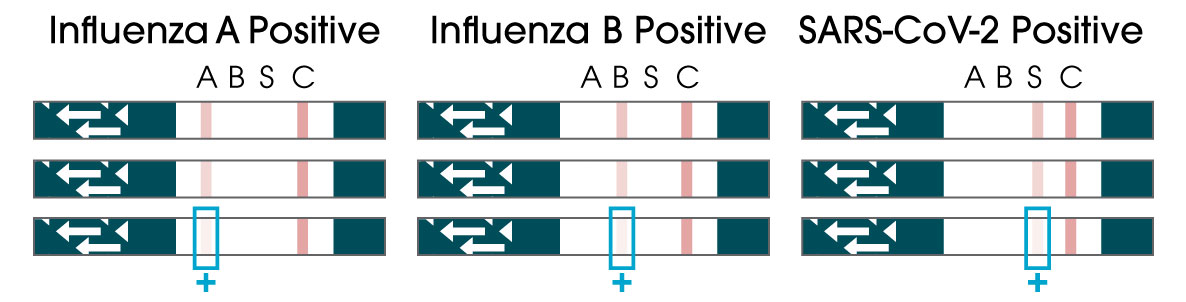
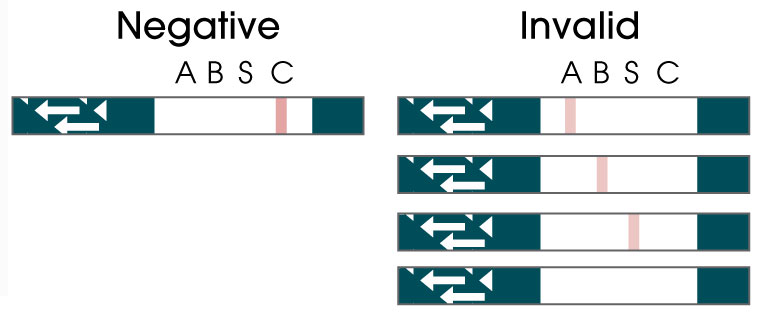
Package Insert
In the USA, this product has not been FDA cleared or approved, but has been authorized by FDA under an Emergency Use Authorization. This product has been authorized only for the detection of proteins from SARS-CoV-2, influenza A and influenza B, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.

From only one sample, the OSOM® Flu SARS-CoV-2 Combo Test simultaneously detects and differentiates between COVID-19, Flu A, and Flu B, allowing healthcare providers to make more informed decisions when treating patients who are symptomatic for common viral infections.
- Rapid: Differentiate between three viral infections (COVID-19, Flu A, & Flu B) in only 10 minutes at the point-of-care.
- Reliable: Proven clinical performance to help you reduce the risk of misdiagnosis and inappropriate treatment.
- Cost Efficient: Unique QC Inside® feature that provides you with two additional tests for external quality control testing.
- Easy-to-Use: Ready-to-use extraction buffers contained in each sample collection tube, so you do not have to perform a rehydration or dispensing step.
- Peace-of-Mind: Manufactured in the USA so you can avoid delays from international product shipments.
Sample Type: Anterior nasal sample




Package Insert
In the USA, this product has not been FDA cleared or approved, but has been authorized by FDA under an Emergency Use Authorization. This product has been authorized only for the detection of proteins from SARS-CoV-2, influenza A and influenza B, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
OSOM - Covid-19, Flu A & B Combo Test, 25ct [ MP977-1080 ]
|
Price: $417.99
|
|
